Protein Engineering
Protein Engineering
Designing the Future of Biotechnology and Medicine
Protein engineering is a transformative field within biotechnology
that involves the design, modification, and construction of
proteins with improved or novel functions. It plays a crucial role in
advancing therapeutics, industrial processes, and agricultural innovations by
tailoring proteins to meet specific functional demands. Through a combination
of molecular biology, computational modelling, and synthetic biology,
scientists are redefining what proteins can do.
In an era where biological solutions are increasingly sought
for complex problems—ranging from chronic diseases to climate change—protein
engineering is at the forefront of innovation. This article explores the
science, methods, applications, and future potential of protein engineering,
providing a structured guide for students, researchers, and industry
professionals alike.
The Science Behind Protein Engineering and Its Core
Techniques
At its essence, protein engineering involves
manipulating a protein’s amino acid sequence to change its structure and
function. Proteins are chains of amino acids that fold into specific
three-dimensional shapes, which determine their biological roles. By modifying
these sequences, scientists can enhance stability, activity, or specificity, or
even create entirely new functions.
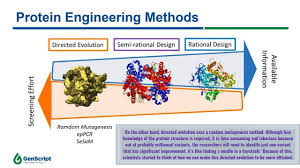
There are two main strategies in protein engineering:
rational design and directed evolution. Rational design relies on
detailed knowledge of a protein’s structure and function, often using
computational tools to predict beneficial changes. Directed evolution, on the
other hand, mimics natural selection in the lab. It involves introducing random
mutations into a protein’s gene, expressing the variants, and selecting those
with improved traits.
Tools like X-ray crystallography, nuclear magnetic
resonance (NMR), and cryo-electron microscopy are employed to
determine protein structures, while bioinformatics and molecular
dynamics simulations assist in predicting outcomes of modifications.
Techniques such as site-directed mutagenesis, phage display, and CRISPR-Cas
systems are routinely used to implement genetic changes.
Institutions like Protein
Data Bank (PDB) and CSIRO’s
Synthetic Biology Future Science Platform provide essential resources and
research infrastructure supporting protein engineering efforts in Australia and
globally.
Applications in Therapeutics and Drug Design
One of the most significant impacts of protein
engineering is in therapeutics. Engineered proteins are at the heart
of many biologic drugs, including monoclonal antibodies, insulin
analogues, and enzyme replacement therapies. These proteins are customized
for optimal performance in the human body, ensuring increased efficacy, longer
half-life, and reduced immunogenicity.
For example, monoclonal antibodies used in cancer
immunotherapy are engineered to target specific antigens on tumor cells
while minimizing effects on healthy tissue. Fusion proteins and antibody-drug
conjugates are further refinements made possible by precise engineering
techniques.
In the case of genetic diseases such as cystic fibrosis
or hemophilia, engineered proteins can compensate for defective or
missing enzymes, dramatically improving patient outcomes. The emerging field of
protein therapeutics continues to expand with innovations like bispecific
antibodies, which can bind two different targets simultaneously.
Australia’s Therapeutic
Goods Administration (TGA) plays a key role in regulating protein-based
drugs, ensuring safety and efficacy through rigorous clinical trial and
approval processes.
Furthermore, protein engineering aids in vaccine
development. The mRNA vaccines developed for COVID-19 were based on a modified
spike protein sequence designed for higher stability and immune response—a
prime example of rational design applied to global health.
Industrial and Environmental Applications
Beyond medicine, protein engineering has revolutionized
numerous industrial and environmental sectors. Enzymes engineered for
specific catalytic functions are widely used in chemical manufacturing, biofuel
production, food processing, and waste treatment.
In the textile and detergent industries, thermostable and
pH-resistant enzymes have replaced harsh chemicals, offering environmentally
friendly alternatives. Proteins such as cellulases, proteases,
and lipases are tailored for use under industrial conditions, improving
process efficiency and sustainability.
In agriculture, engineered proteins are used to create crops
resistant to pests, diseases, and environmental stresses. For instance, the but
toxin protein, derived from Bacillus thuringiensis, has been engineered
and expressed in genetically modified plants to deter insect pests, reducing
the need for chemical pesticides.
Additionally, environmental bioremediation benefits from
proteins designed to break down pollutants such as plastics, petroleum, and
heavy metals. These biocatalysts offer potential solutions to global pollution
challenges and support circular economy initiatives.
In Australia, research institutions like Monash University and University of Queensland lead efforts in
green biotechnology and enzyme design, addressing both climate and resource
challenges.
Challenges and Ethical Considerations in Protein
Engineering
Despite its remarkable capabilities, protein engineering
faces several challenges. Protein folding is complex and difficult to predict,
especially for large proteins or those requiring post-translational
modifications. Incorrect folding can lead to loss of function or even toxic
effects in therapeutic applications.
Scalability is another concern. Producing engineered
proteins at industrial scale while maintaining quality and functionality
requires sophisticated bioprocessing systems and infrastructure.
Regulatory hurdles must also be addressed, particularly when
engineered proteins are introduced into humans, animals, or the environment.
Comprehensive testing, biosafety evaluation, and regulatory approval are
time-intensive but essential steps.
Ethically, the manipulation of biological systems raises
questions about the boundaries of human intervention in nature. While the goal
is often to improve health or environmental outcomes, concerns about unintended
consequences, bioterrorism, or inequitable access to these technologies
persist.
Moreover, the patenting of engineered proteins and genetic
sequences can lead to monopolization and limit access to life-saving therapies.
Efforts to balance intellectual property rights with public interest are
ongoing in policy and academic circles.
Frameworks from OECD and
local ethics committees guide responsible innovation in protein engineering,
encouraging transparency, collaboration, and inclusivity in biotech
development.
The Future of Protein Engineering: Synthetic Biology and
AI Integration
The future of protein engineering lies at the
intersection of synthetic biology, artificial intelligence (AI),
and machine learning. These disciplines are converging to create
powerful tools for designing and optimizing proteins with unprecedented
precision.
AI-driven platforms like AlphaFold have made accurate
protein structure prediction more accessible, significantly accelerating the
design phase of engineering projects. Machine learning models trained on vast
protein datasets can predict the impact of mutations on stability and function,
reducing the need for trial-and-error experimentation.
Synthetic biology allows for the creation of entirely new
proteins not found in nature—de novo proteins—with bespoke functions
tailored to specific industrial or medical needs. These custom-built proteins
expand the boundaries of what’s possible, moving from tweaking natural
sequences to inventing new ones.
Looking ahead, protein engineering is expected to
play a central role in tackling global issues such as antimicrobial resistance,
food security, and chronic disease management. It also holds promise in
regenerative medicine, including tissue engineering and organ repair, by
designing proteins that guide cell growth and differentiation.
Australia’s continued investment in biotechnology,
through agencies like Bio platforms
Australia, ensures that local researchers and industries remain at the
cutting edge of these developments.
FAQ
Q1: What is the difference between rational design and
directed evolution in protein engineering?
Rational design uses known structural and functional data to make targeted
changes to a protein, while directed evolution introduces random mutations and
selects for desired traits through multiple iterations.
Q2: Are engineered proteins safe for use in humans?
Yes, but only after extensive testing. Engineered proteins used in therapeutics
undergo rigorous preclinical and clinical trials, with approval from regulatory
agencies like the TGA to ensure safety and efficacy.
Q3: Can protein engineering be used outside of
healthcare?
Absolutely! It’s widely used in industries such as food, textiles, biofuels,
and environmental clean-up. Enzymes engineered for specific tasks improve
efficiency and sustainability across various sectors.
Read related blogs:
#proteinengineering, #biotechinnovation, #syntheticbiology,
#rationaldesign, #directedevolution, #engineeredproteins, #proteintherapeutics,
#enzymeengineering, #industrialbiotechnology, #biomedicalengineering,
#moleculardesign, #alphafold, #drugdevelopment, #greenbiotechnology,
#bioscienceresearch

Comments
Post a Comment